Our research laboratory is interested in the development of novel supramolecular nanomaterials and technologies to assist the fundamental understanding of immunological mechanisms hidden behind challenging diseases, such as cancer, atherosclerosis, myocardial infarction, etc.
Supramolecular strategies are employed to regulate the biological processes to find out new therapeutic targets and new therapeutic approaches. A final complementary effort in the laboratory focuses on developing new formulations to monitor and manipulate immune cells, guiding future intervention strategies through new fundamental insights into the function of the immune system, finally achieving satisfactory therapeutic benefits in the clinic.
Recent three main research area:
1. Precise supramolecular theranostics.
2. Supramolecular regulation of tumor microenvironment and tumor metabolism for cancer immunotherapy.
3. mRNA delivery systems and vaccines.
The core background of our lab is the connection between supramoleular chemistry and human health.
We hope to develop novel theranostic platforms using non-covalent interactions, moving the obstacles faced by the traditional therapeutics on their way to clinical trials. We firstly define the concept of supramolecular theranostics, and have constructed several supramolecular systems already
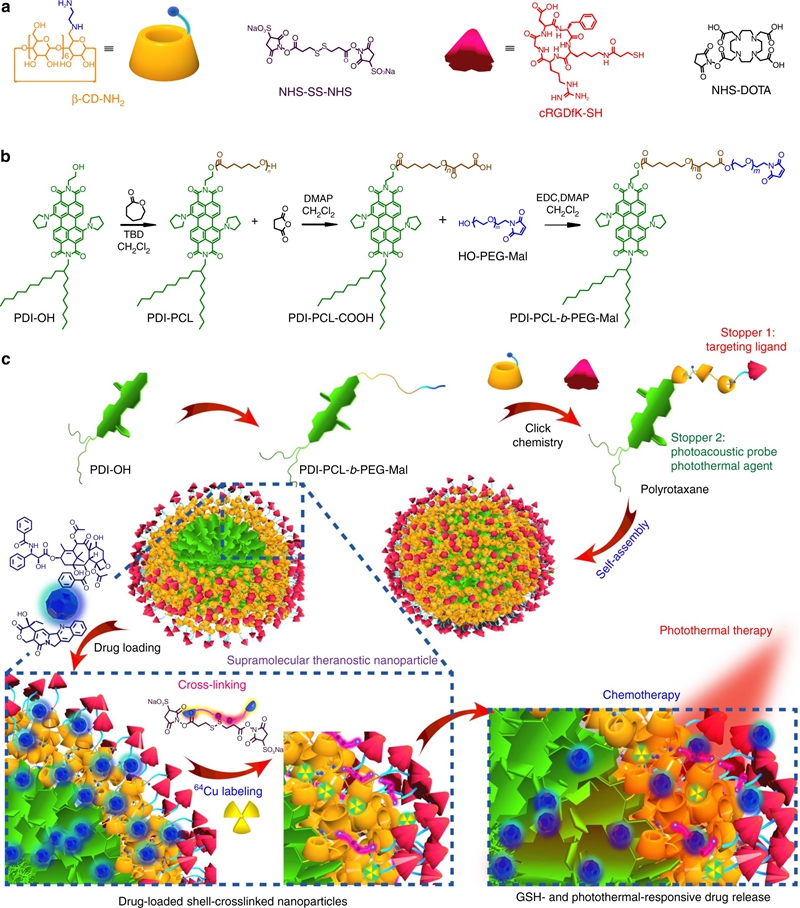
The development of smart theranostic systems with favourable biocompatibility, high loading efficiency, excellent circulation stability, potent anti-tumour activity, and multimodal diagnostic functionalities is of importance for future clinical application. The premature burst release and poor degradation kinetics indicative of polymer-based nanomedicines remain the major obstacles for clinical translation. Herein we prepare theranostic shell-crosslinked nanoparticles (SCNPs) using a β-cyclodextrin-based polyrotaxane (PDI-PCL-b-PEG-RGD⊃β-CD-NH2) to avoid premature drug leakage and achieve precisely controllable release, enhancing the maximum tolerated dose of the supramolecular nanomedicines. cRGDfK and perylene diimide are chosen as the stoppers of PDI-PCL-b-PEG-RGD⊃β-CD-NH2, endowing the resultant SCNPs with excellent integrin targeting ability, photothermal effect, and photoacoustic capability. In vivo anti-tumour studies demonstrate that drug-loaded SCNPs completely eliminate the subcutaneous tumours without recurrence after a single-dose injection combining chemotherapy and photothermal therapy. These supramolecular nanomedicines also exhibit excellent anti-tumour performance against orthotopic breast cancer and prevent lung metastasis with negligible systemic toxicity.
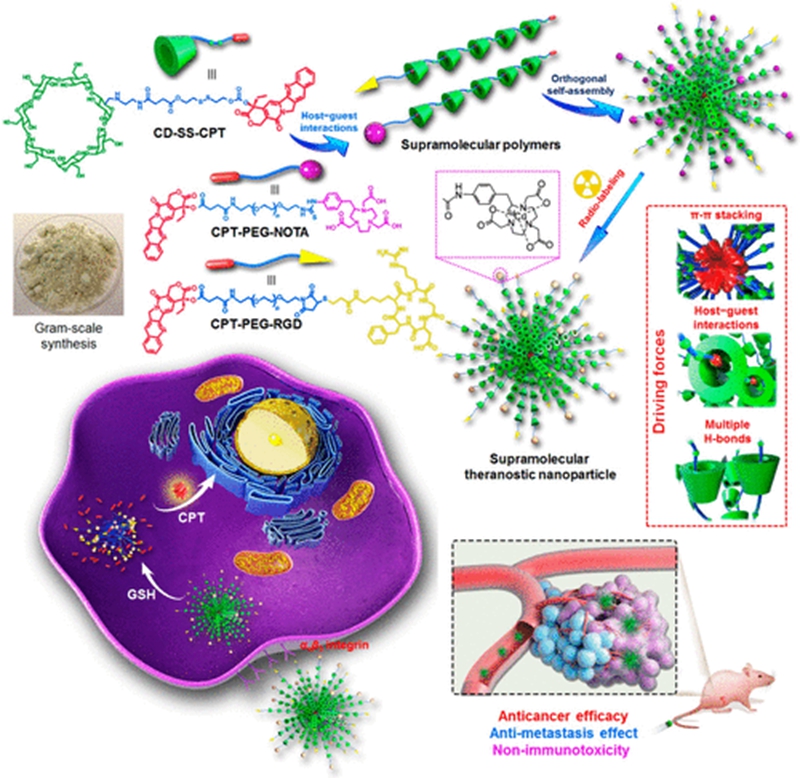
Nanomedicines have achieved several breakthroughs in cancer treatment over the past decades; however, their potential immunotoxicities are ignored, which results in serious adverse effects and greatly reduces the potential in clinical translation. Herein, we innovatively develop a theranostic supramolecular polymer using β-cyclodextrin as the host and camptothecin (CPT) as the guest linked by a glutathione-cleavable disulfide bond. The supramolecular polymerization remarkably increases the solubility of CPT by a factor of 232 and effectively inhibits its lactone ring opening in physiological environment, which is favorable for intravenous formulation and maintenance of the therapeutic efficacy. Supramolecular nanoparticles can be prepared through orthogonal self-assembly driven by π–π stacking interaction, host–guest complexation, and hydrogen bonds. The sophisticated nanomedicine constructed from the obtained supramolecular polymer can be specifically delivered to tumor sites and rapidly excreted from body after drug release, thus effectively avoiding systemic toxicity, especially long-term immunotoxicity. In vivo investigations demonstrate this supramolecular nanomedicine possesses superior antitumor performance and antimetastasis capability. This pioneering example integrating the advantages of the dynamic nature of supramolecular chemistry and nanotechnology provides a promising platform for cancer theranostics.
In near future, we hope to further dig in the preparation of novel delivery systems to develop vaccines for cancer and other diseases. We will also develop supramolecular theranostics using host-guest chemistry using artificial macrocycles and biomacromolecules, which are very interesting and meaningful.

Quick links: Research | Members | Publications | News| Gallery | Join Us
Useful links: Tsinghua | Tsinghua Chem | Tsinghua Library | Web of Science | Science I Nature I Cell I ACS